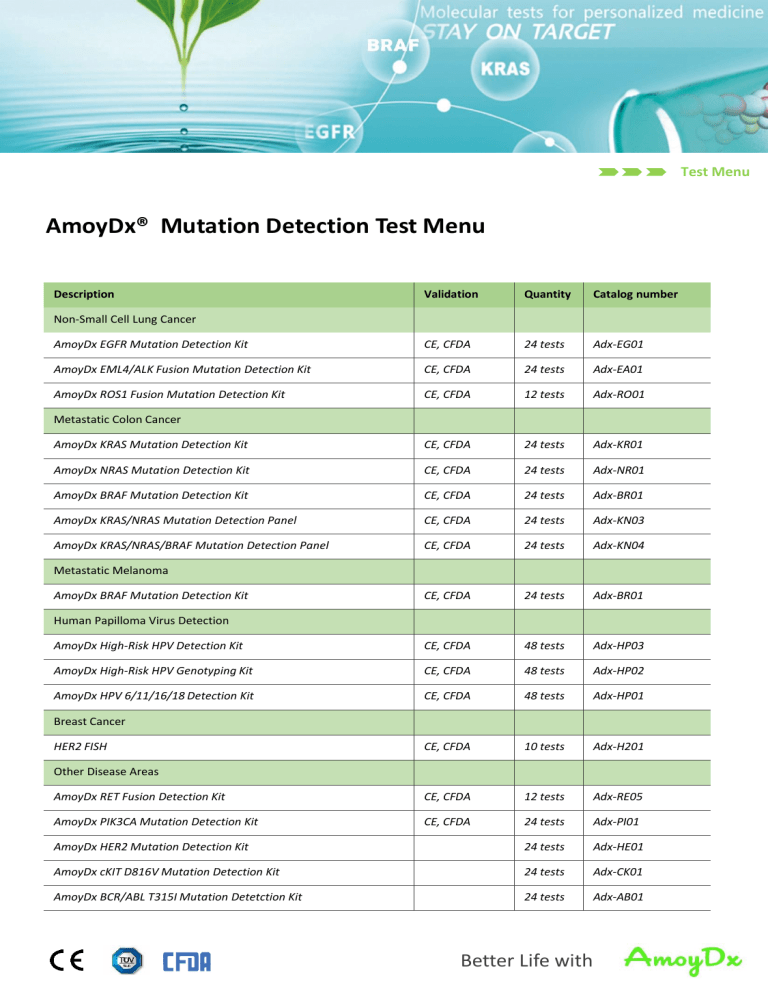
Test Menu
AmoyDx® Mutation Detection Test Menu
Description
Validation
Quantity
Catalog number
AmoyDx EGFR Mutation Detection Kit
CE, CFDA
24 tests
Adx-EG01
AmoyDx EML4/ALK Fusion Mutation Detection Kit
CE, CFDA
24 tests
Adx-EA01
AmoyDx ROS1 Fusion Mutation Detection Kit
CE, CFDA
12 tests
Adx-RO01
AmoyDx KRAS Mutation Detection Kit
CE, CFDA
24 tests
Adx-KR01
AmoyDx NRAS Mutation Detection Kit
CE, CFDA
24 tests
Adx-NR01
AmoyDx BRAF Mutation Detection Kit
CE, CFDA
24 tests
Adx-BR01
AmoyDx KRAS/NRAS Mutation Detection Panel
CE, CFDA
24 tests
Adx-KN03
AmoyDx KRAS/NRAS/BRAF Mutation Detection Panel
CE, CFDA
24 tests
Adx-KN04
CE, CFDA
24 tests
Adx-BR01
AmoyDx High-Risk HPV Detection Kit
CE, CFDA
48 tests
Adx-HP03
AmoyDx High-Risk HPV Genotyping Kit
CE, CFDA
48 tests
Adx-HP02
AmoyDx HPV 6/11/16/18 Detection Kit
CE, CFDA
48 tests
Adx-HP01
CE, CFDA
10 tests
Adx-H201
AmoyDx RET Fusion Detection Kit
CE, CFDA
12 tests
Adx-RE05
AmoyDx PIK3CA Mutation Detection Kit
CE, CFDA
24 tests
Adx-PI01
AmoyDx HER2 Mutation Detection Kit
24 tests
Adx-HE01
AmoyDx cKIT D816V Mutation Detection Kit
24 tests
Adx-CK01
AmoyDx BCR/ABL T315I Mutation Detetction Kit
24 tests
Adx-AB01
Non-Small Cell Lung Cancer
Metastatic Colon Cancer
Metastatic Melanoma
AmoyDx BRAF Mutation Detection Kit
Human Papilloma Virus Detection
Breast Cancer
HER2 FISH
Other Disease Areas
Better Life with
AmoyDx® DNA/RNA Isolation Kits
Description
Quantity
Catalog number
AmoyDx FFPE DNA Isolation Kit
36 tests
Adx-FF01
AmoyDx Tissue DNA Kit
36 tests
Adx-TI01
AmoyDx Cell DNA Kit
36 tests
Adx-CE01
AmoyDx Blood DNA Kit
36 tests
Adx-BL01
AmoyDx Serum/Plasma Cell-free DNA Kit
24 tests
Adx-BL02
AmoyDx FFPE RNA Isolation Kit
36 tests
Adx-FF04
AmoyDx FFPE DNA/ RNA Isolation Kit
36 tests
Adx-FF03
AmoyDx Tissue DNA/RNA Kit
36 tests
Adx-TI03
DNA Isolation Kits
RNA Isolation Kits
Workflow of AmoyDx® Mutation Detection Tests
Tissue collection
DNA isolation
3-4 hours
Result
Realtime PCR Set-up
0,5 hours
Clinical implementation
Wild Type
Appropriate Guideline
Mutant
Appropriate Guideline
Invalid
Repeat test
1,5 hours
> 8 hours
Gen Era Diagnostik Sağlık Hizmetleri. A.Ş.
My Office İş Merkezi Bölüm 19 Barbaros Mah.
Çiğdem Sok. No:1 34746 Batı Ataşehir İstanbul
Tel. +90 216 455 58 22-23 Fax. +90 216 455 58 24
E-mail. info@gen-era.com.tr Url. www.gen-era.com.tr
Test menu
